
Independent Research
36) Mrinmay Baidya and Suman De Sarkar* "Synthesis of Quinoxalines through Cu-electrocatalytic Azidation/Annulation Cascade at Low Catalyst Loading" Org. Lett. 2023, 25, 31, 5896–5901 [Link]


35) Atreyee Halder,‡Sayan Ghosh‡, and Suman De Sarkar* "Transition Metal and Base‐free Electro‐oxidative Regioselective Trifluoromethylation of Imidazo [1, 2‐a] pyridines" Asian J. Org. Chem., 2023, 12, e202300294 [Link]


34) Mrinmay Baidya, Jhilik Dutta and Suman De Sarkar* "Electrochemical Organoselenium Catalysis for the Selective Activation of Alkynes: Easy Access to Carbonyl-pyrroles/oxazoles from N‑Propargyl Enamines/Amides" Org. Lett. 2023, 25, 3812 [Link]


33) Kingshuk Mahanty, Suman Kumar Saha, Atreyee Halder and Suman De Sarkar* "Mediator-free electrochemical trifluoromethylation: a cascade approach for the synthesis of trifluoromethylated isoxazolines" Chem. Commun. 2023, 59, 4467 [Link]


32) Sudhir Kumar Hota, Satya Prakash Panda, Sanju Das, Sanat Kumar Mahapatra, Lisa Roy*, Suman De Sarkar*, and Sandip Murarka* "Photoinduced Electron Donor–Acceptor Complex-Mediated Radical Cascade Involving N-(Acyloxy)phthalimides: Synthesis of Tetrahydroquinolines" J. Org. Chem. 2023, 88, 2543 [Link]


31) Kingshuk Mahanty, Atreyee Halder and Suman De Sarkar* "Synthesis of N-Sulfonyl Amidines and 1,3,4-Oxadiazoles through Electrochemical Activation of DMF" Advanced synthesis and catalysis 10.1002/adsc.202201019 [Link]


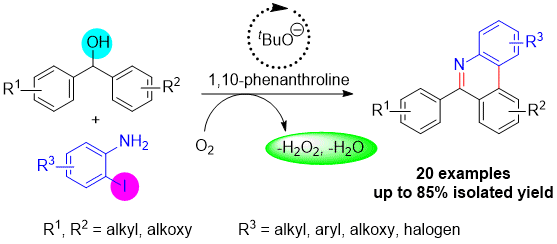
30) Tanumoy Mandal, Samrat Mallick, Nidhi Kumari, and Suman De Sarkar* "Visible-Light-Mediated Synthesis of Phenanthrenes through Successive Photosensitization and Photoredox by a Single Organocatalyst" Organic Letters 10.1021/acs.orglett.2c03612 [Link]


29) Sayan Ghosh‡, Jhilik Dutta‡, Atreyee Halder, and Suman De Sarkar* "Easy Access to N-(Pyridin-2-yl)benzamides through Electro-oxidative Ring Opening of 2-Arylimidazo[1,2-a]pyridines" Synlett DOI: 10.1055/a-1956-9993 [Link]


28) Atreyee Halder, Kingshuk Mahanty, Debabrata Maiti, and Suman De Sarkar* "Recent Developments in the Electro-reductive Functionalization of C−halogen Bonds" Synthesis DOI: 10.1055/a-1944-9494 [Link]


27) Mrinmay Baidya, Samrat Mallick, and Suman De Sarkar* "Regioselective Synthesis of N2-Aryl 1,2,3-Triazoles via Electro-oxidative Coupling of Enamines and Aryldiazonium Salts" [Link]


26) Mrinmay Baidya, Debabrata Maiti, Lisa Roy*, Suman De Sarkar* "Trifluoroethanol as a Unique Additive for the Chemoselective Electrooxidation of Enamines to Access Unsymmetrically Substituted NH-Pyrroles." [Link]


25) Sanju Das,Tanumoy Mandal,Suman De Sarkar* "Acridine Orange Hemi(Zinc Chloride) Salt as a Lewis Acid-Photoredox Hybrid Catalyst for the Generation of α-Carbonyl Radicals" [Link]


24) Tanumoy Mandal, Aznur Azim, Sanju Das, and Suman De Sarkar* "Organophotoredox Catalyzed Stereoselective Nitration of Olefins with tert-Butyl Nitrite under Air" [Link]


23) Sanju Das, Aznur Azim, Sudhir Kumar Hota, Satya Prakash Panda, Sandip Murarka* and Suman De Sarkar* "Organophotoredox-catalyzed redox-neutral cascade involving N-(acyloxy)phthalimides and allenamides: Synthesis of indoles" Chem. Commun. 2021, 57,13130. [link]


22) Raki Mandal, Kingshuk Mahanty, Subhendu Mandal, Suman De Sarkar* and Pradip K. Tarafdar* "Membrane transport inspired hydrolysis of non-activated esters at near physiological pH "Chem. Commun. 2021, 57, 11088. [link]


21) Debabrata Maiti, Atreyee Halder, Aswathy Sasidharan Pillai, and Suman De Sarkar* "Synthesis of Polysubstituted Furans through Electrochemical Selenocyclization of Homopropargylic Alcohols" J. Org. Chem. 2021, 86, 16084.[Link]


20) Atreyee Halder, Kingshuk Mahanty, Debabrata Maiti and Suman De Sarkar* "Highly Diastereoselective Synthesis of Dihydro-benzoimidazo-[1,3]-thiazines via Electro-oxidative Selenocyclization of Thioallyl Benzoimidazoles" Chem. Asian J. 2021, 16, 3895. [Link]

19) Sanju Das, Sushanta Kumar Parida,‡ Tanumoy Mandal,‡ Sudhir Kumar Hota, Lisa Roy, * Suman De Sarkar * and Sandip Murarka "Manganese-catalyzed Electro-oxidative Azidation-annulationCascade to Access Oxindoles and Quinolinones" Org. Chem. Front. 2021, 8, 2256 [Link]


18) Debabrata Maiti, Kingshuk Mahanty, and Suman De Sarkar* "Manganese-catalyzed Electro-oxidative Azidation-annulationCascade to Access Oxindoles and Quinolinones" Chem Asian J. 2021, 16, 748 – 752 (Joint 1st author) [Link]

17) Debabrata Maiti, Kingshuk Mahanty, and Suman De Sarkar* "Manganese-Catalyzed Electrochemical Tandem Azidation–Coarctate Reaction: Easy Access to 2-Azo-benzonitriles" Org. Lett. 2021, 23, 1742−1747 (Joint 1st author) [Link]


16) Sushanta Kumar Parida, Tanumoy Mandal, Sanju Das, Sudhir Kumar Hota, Suman De Sarkar,* and Sandip Murarka* "Single Electron Transfer-Induced Redox Processes Involving N‑(Acyloxy)phthalimides" ACS Catal. 2021, 11, 1640−1683 (Joint 1st author) [Link]


15) Ayan Bhattacharyya, Suman De Sarkar,* and Anindita Das* "Supramolecular Engineering and Self-Assembly Strategies in Photoredox Catalysis" ACS Catal. 2021, 11, 710−733 [Link]


14) V. Arun, Lisa Roy* and Suman De Sarkar* "Alcohols as Fluoroalkyl Synthons: Ni-catalyzed Dehydrogenative Approach to Access Polyfluoroalkyl Bis-indoles" Chem. Eur.J. 2020 , 26,16649 –16654 [Link]

13) Kingshuk Mahanty, Debabrata Maiti, Suman De Sarkar, "Regioselective C–H Sulfonylation of 2H-Indazoles by Electrosynthesis" J. Org. Chem. 2020, 85, 5, 3699–3708 (Joint 1st author) [Link]


12) Atreyee Halder, Debabrata Maiti, Suman De Sarkar, "Mechanochemical Synthesis of Functionalized Quinolines by Iodine Mediated Oxidative Annulation" Chem Asian J. 2020, 15, 577 – 580 [Link]

11) Sanju Das, Sushanta Kumar Parida, Tanumoy Mandal, Laxmikanta Sing, Suman De Sarkar* and Sandip Murarka* "Organophotoredox Catalyzed Cascade Radical Annulation of 2-(Allyloxy)arylaldehydes with N-(acyloxy)phthalimides: Towards Alkylated Chroman-4-one derivatives" Chem Asian J. 2020, 15, 568 – 572 [Link]

10) S. Mallick, M. Baidya, K. Mahanty, D. Maiti, S. De Sarkar, "Electrochemical Chalcogenation of β,γ-Unsaturated Amides and Oximes to Corresponding Oxazolines and Isoxazolines" Adv. Synth. Catal. 2020, 362, 1046 – 1052. [Link]


9) D. Maiti, A. Halder, S. De Sarkar, "Base‐Promoted Aerobic Oxidation/Homolytic Aromatic Substitution Cascade toward the Synthesis of Phenanthridines" Adv. Synth. Catal. 2019, 361, 4941 – 4948. [Link] (Selected as VIP)

8) S. Das, S. Mallick, S. De Sarkar, "Cobalt-Catalyzed Sustainable Synthesis of Benzimidazoles by Redox-Economical Coupling of o-Nitroanilines and Alcohols" J. Org. Chem. 2019, 84, 12111−12119. [Link]


7) V. Arun, S. De Sarkar, "Recent Developments in the de Novo Synthesis of Heterocycles by First-Row Transition-Metal-Catalyzed Acceptorless Dehydrogenation" Cur. Org. Chem. 2019, 23, 1005.[Link]


6) T. Mandal, S. Das, S. De Sarkar, "Nickel(II) Tetraphenylporphyrin as an Efficient Photocatalyst Featuring Visible Light Promoted Dual Redox Activities" Adv. Synth. Catal. 2019, 361, 3200.[Link]


5) V. Arun, K. Mahanty, S. De Sarkar, "Nickel‐Catalyzed Dehydrogenative Couplings" Chem CatChem. 2019, 11, 2243.[Link] (Joint 1st author)


4) M. Ghosh, S. De Sarkar, "meta‐ and para‐Selective C−H Functionalization using Transient Mediator and Noncovalent Template" Asian J. Org. Chem. 2018, 7, 1236.[Link]

3) S. Das, D. Maiti, S. De Sarkar, "Synthesis of Polysubstituted Quinolines from α-2-Aminoaryl Alcohols Via Nickel-catalyzed Dehydrogenative Coupling" J. Org. Chem. 2018, 83, 2309.[Link]


2) S. Dey, S. De Sarkar, "Synthetic Applications of Vinyl Ruthenium Carbene Derived from Diazoalkanes and Alkynes" Adv. Synth. Catal. 2017, 359, 2709. [Link]


1) S. De Sarkar, "Remote C−H Functionalization by a Palladium-Catalyzed Transannular Approach" Angew. Chem., Int. Ed. 2016, 55, 10558. [Link]


Doctoral and Postdoctoral Research:
17) J. Li, K. Korvorapun, S. De Sarkar, T. Rogge, D. J. Burns, S. Warratz, L. Ackermann, Nature Commun. 2017, 8, 15430. [Link] (Joint 1st author)
16) S. De Sarkar, N. Y. P. Kumar and L. Ackermann, Chem. Eur. J. 2017, 23, 84. [Link]
15) S. De Sarkar, J. F. Blom, Y. Bethuel, F. Jüttner, K. Gademann, Helv. Chim. Acta. 2016, 99, 760. [Link]
14) J. Li, S. De Sarkar and L. Ackermann, Top. Organomet. Chem. 2016, 55, 217. [Link]
13) J. Li, S. Warratz, D. Zell, S. De Sarkar, E. E. Ishikawa, L. Ackermann, J. Am. Chem. Soc. 2015, 137, 13894. [Link]
12) S. De Sarkar and L. Ackermann, Chem. Eur. J. 2014, 20, 13932. [Link]
(Most Accessed Article 09-2014 to 08-2015 and Selected for ChemInform Abstract 2015 by the Editors)
11) S. De Sarkar, W. Liu, S. I. Kozhushkov and L. Ackermann, Adv. Synth. Catal. 2014, 356, 1461. [Link]
(>174 Citations)
10) R. C. Samanta, S. De Sarkar, R. Fröhlich, S. Grimme and A. Studer, Chem. Sci. 2013, 4, 2177. [Link]
9) S. De Sarkar, A. Biswas, R. C. Samanta and A. Studer, Chem. Eur. J. 2012, 19, 4664. [Link]
(>114 Citations and selected for ChemInform Abstract 2013 by the Editors)
8) A. Biswas, S. De Sarkar, L. Tebben and A. Studer, Chem. Commun. 2012, 48, 5190. [Link]
7) R. C. Samanta, B. Maji, S. De Sarkar, K. Bergander, R. Fröhlich, C. M.-Lichtenfeld, H. Mayr and A. Studer, Angew. Chem., Int. Ed. 2012, 51, 5234. [Link]
6) A. Biswas, S. De Sarkar and A. Studer, Org. Lett. 2011, 13, 4966. [Link]
(Selected for ChemInform Abstract 2012 by the editors)
5) S. De Sarkar, A. Biswas, C. H. Song and A. Studer, Synthesis 2011, 12, 1974. [Link]
4) S. De Sarkar and A. Studer, Angew. Chem., Int. Ed. 2010, 49, 9266. [Link]
(>143 Citations and highlighted in Synfacts 2011, 96)
3) S. De Sarkar and A. Studer, Org. Lett. 2010, 12, 1992. [Link]
(>133 Citations and selected for ChemInform Abstract 2010 by the editors)
2) S. De Sakar, S. Grimme and A. Studer, J. Am. Chem. Soc. 2010, 132, 1190. [Link]
(>185 Citations and highlighted in Synfacts 2010, 359)
1) J. Guin, S. De Sarkar, S. Grimme and A. Studer, Angew. Chem., Int. Ed. 2008, 47, 8727. [Link]
(>134 Citations and highlighted in Synfacts 2009, 26)


